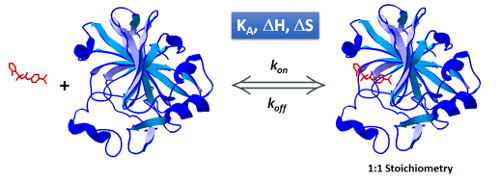
Isothermal Titration Calorimetry (ITC) is the gold standard for the calculation of affinity in molecular interactions. Many times, researchers claim that the high consumption of sample does not offset the use of ITC for Kd calculation.
Conversely, ITC hides many surprises in the acquisition data that can provide more information in a single experiment that other techniques that are more expensive and more complicated to use.
Download the PDF file of Implementation of kinITC into AFFINImeter
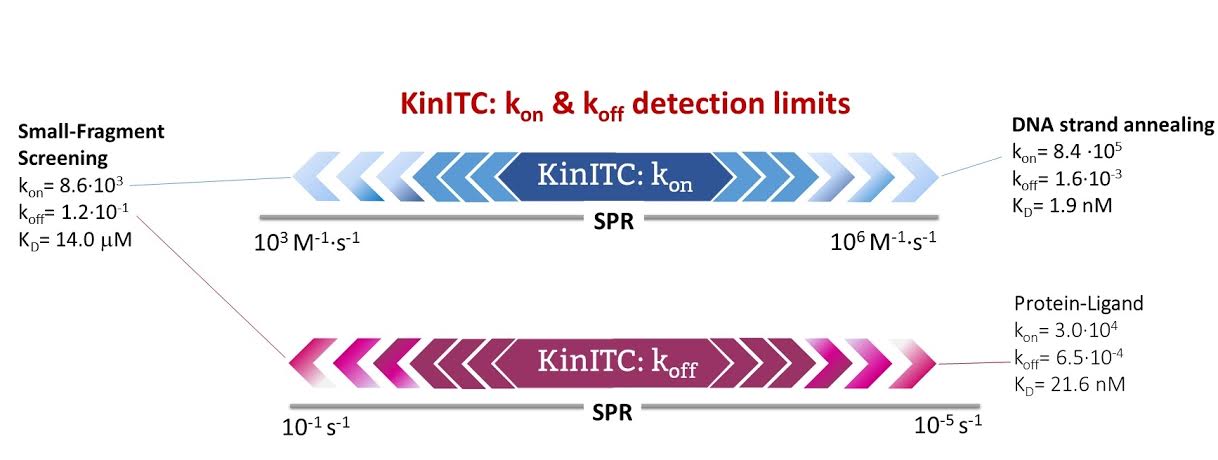
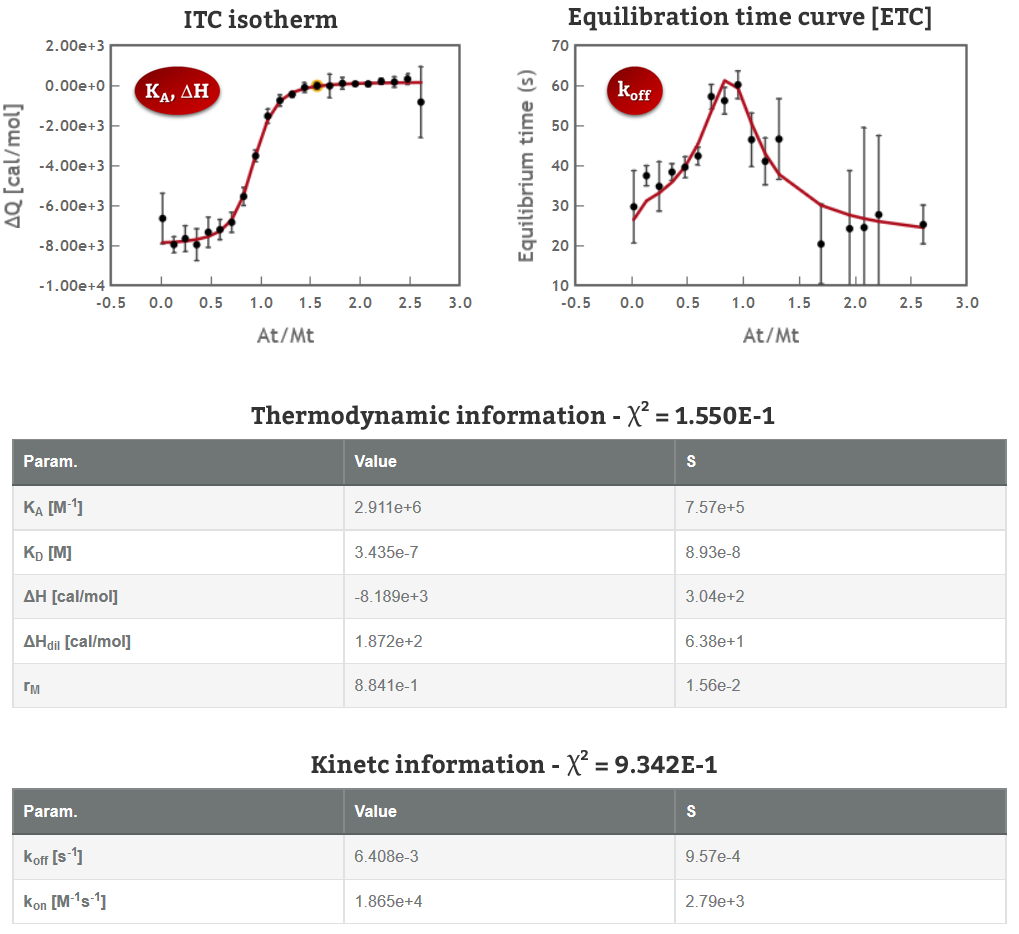
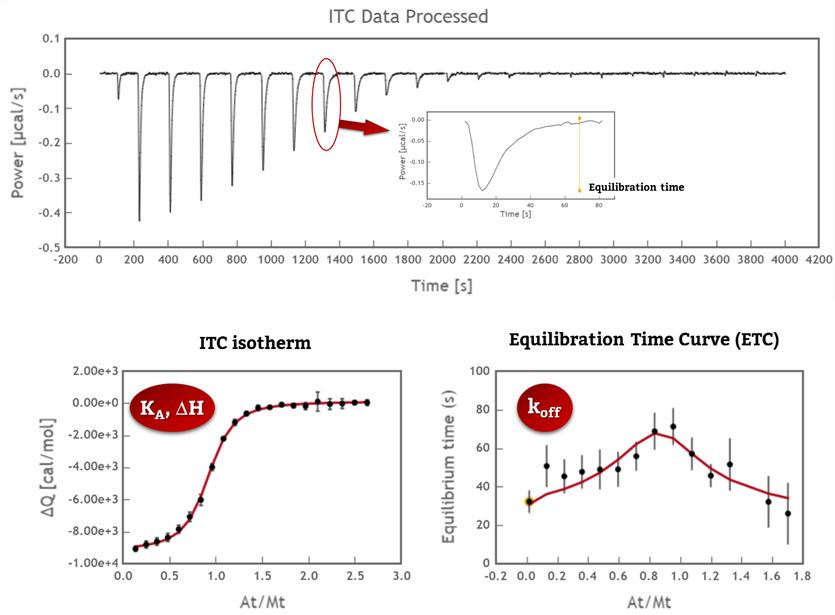
1. ITC collects data from the interaction as a function of time that can be analyzed to obtain kinetic information (kon and koff values). It can cover a very similar range as Surface Plasmon Resonance in a “label-free” and “in-solution” manner (Fig 1).
2. ITC can also provide valuable information about the mechanism of interaction. The high sensitivity of the ITC sensor makes it sensitive to more intriguing interactions as conformational changes, cooperativity…
Find attached a couple of publications describing the application of this new method for ITC data analysis:
Download the PDF file of Implementation of kinITC into AFFINImeter