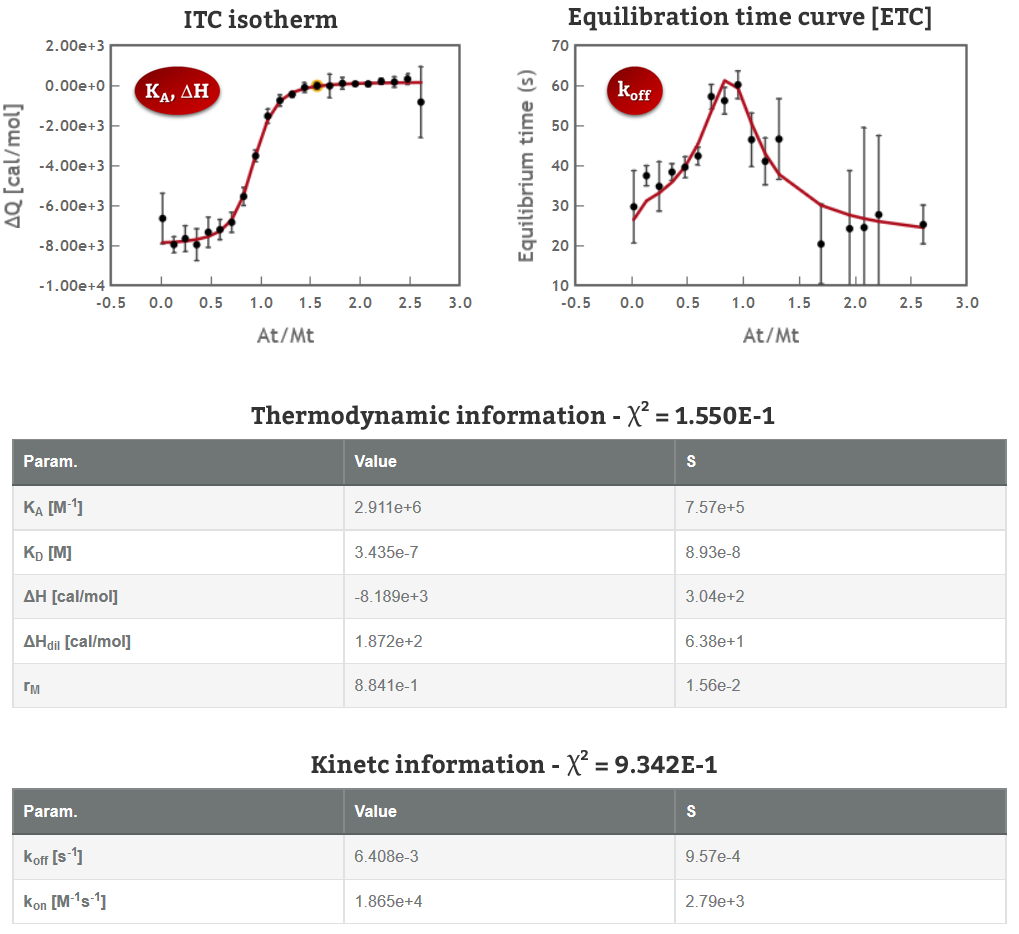
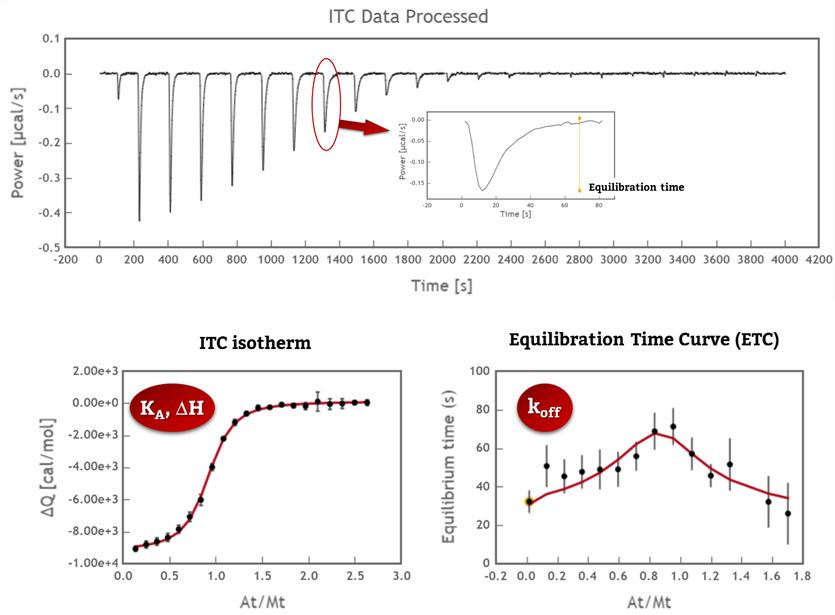
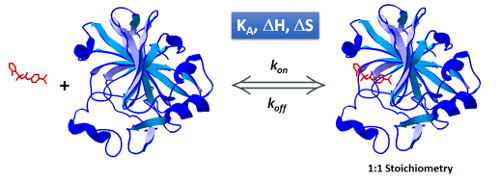
Monitoring a binding event with Isothermal Titration Calorimetry at different temperatures provides a powerful framework for elucidating interesting characteristics of the interaction. Analysis of the isotherms obtained determines the dependence of the association constant (KA) and binding enthalpy (ΔH) with temperature, information that can reveal mechanistic aspects of the interactions, i.e. the existence of allosteric effects and conformational changes (1).
Moreover, kinetic characterization of the interaction at various temperatures gives information about transition state thermodynamics, by means of the dependence of the association and dissociation rate constants (kon and koff) with the temperature. This way, activation free energies of association and dissociation are resolved into its enthalpic and entropic components (2).
Obtaining the full thermodynamic and kinetic profile of 1:1 interactions in a single ITC experiment is now possible with AFFINImeter and KinITC; in order to further exploit the potential of our analytical tools we have recently incorporated a new functionality in AFFINImeter that automatically analyzes variable temperature isothermal Titration Calorimetry assays through Van´t Hoff Plot (Ln(KA) vs 1/T), temperature dependence of ΔH (that determines changes in heat capacity, ΔCp) and Eyring plots (Ln(kon) vs 1/T and Ln(koff) vs 1/T) (3).
AFFINImeter is the only software that provides thermodynamic and kinetic information from a single ITC titration; now incorporates the automatic analysis of variable-temperature experiments.
You can use this feature for free during one month, go to AFFINImeter webpage.
References
- Freiburger L, Auclair K, Mittermaier A. Global ITC fitting methods in studies of protein allostery. Methods 2015, 76, pp 149-161.
- GE Healthcare application note 80. Transition state thermodynamics using Biacore T100, (2007).
- Ladbury, J. and Doyle, M. (2004). Biocalorimetry 2. Chichester: Wiley.