We’ve released AFFINImeter ITC Advanced 1.2.0 for Windows
Do you want to install and use AFFINImeter in your computer? Now you can.
Go ahead and download AFFINImeter ITC for Windows right now (only for 64 bits computers).
Upon download, all users will get a FREE 30 days trial version for AFFINImeter ITC Advanced, including: KinITC, ITC and TA data analysis, Model builder, Design complex fitting and simulation projects for ITC, and a new tool: the rawdata experiments simulator.
Users who purchased a license for the web version have the same license for the Windows version. Just contact us at info@affinimeter.com to activate it.
Release notes for 1.2.0 (Update is recommended):
Important updates have been performed in the windows version of the AFFINImeter-ITC software.
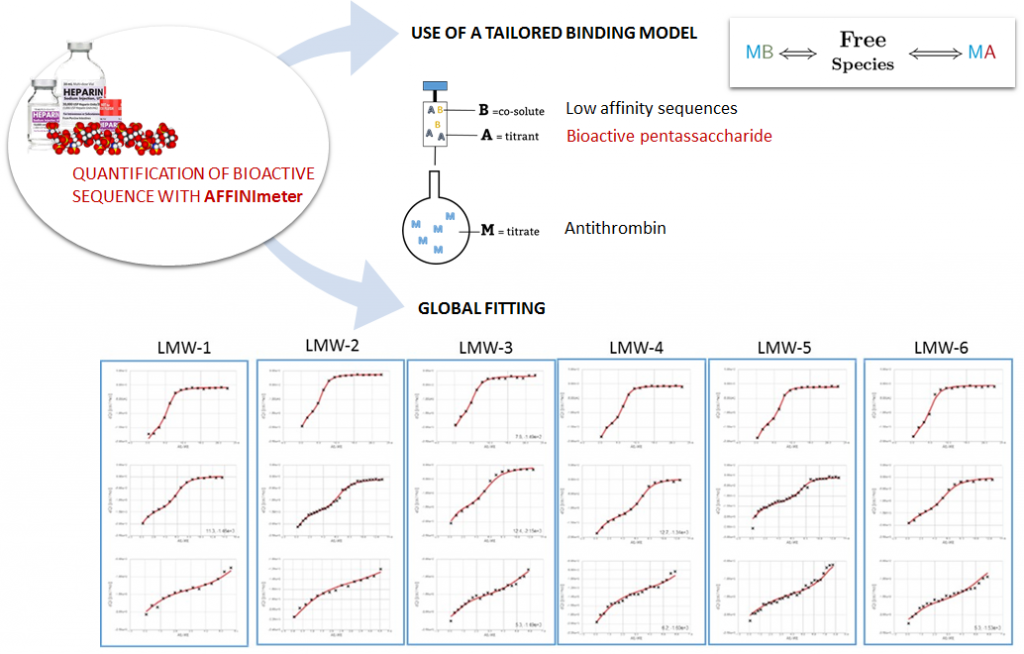
The analysis has implemented the accurate determination of concentrations recently published by Philippe Dumas: (see https://www.biorxiv.org/content/10.1101/512780v1.full).
This update breaks backwards compatibility: this means that all previously created projects (.affprojects) will not be compatible with this version (they will not load in the application or will throw some errors). The previously processed itc/ta raw data should be processed again to update the accurate calculation of concentrations in the equilibrium isotherm.
Other problems/issues fixed:
- [Fixed] Fitting Projects Output may show incorrect results while performing complex fittings.
- [Added] Fitting Projects now have an option to download a ZIP file containing all charts in the project, and also some new and specific ones.
- [Added] Presentation of local minima for fitting projects was improved.
- [Added/Fixed] Y labels, ticks and names
- Y values/ticks will have an exponential format instead of the SI prefixes
- Some Y label names were fixed
- [Fixed] “Auto” setting in KinITC will stay active after deleting points in the Isotherm, if user the user has it active. Previously, after changing/deleting points in the isotherm, that setting was lost.
- [Added] Included more references in the Help Section