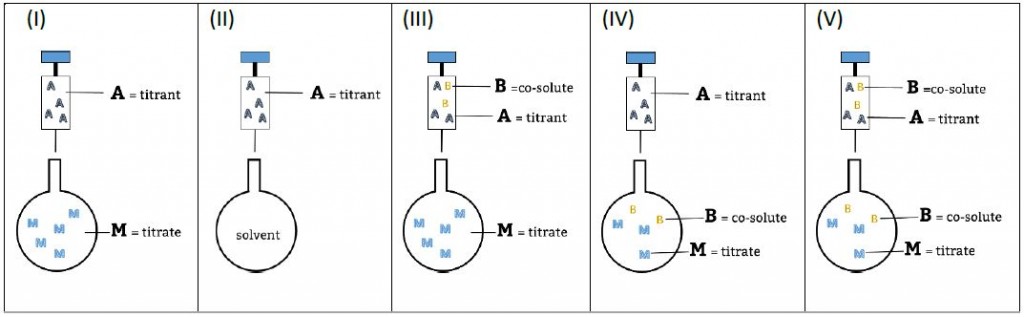
Experimental setups and nomenclature used in AFFINImeter
A few days ago AFFINImeter launched an Isothermal Titration Calorimetry (ITC) data analysis challenge. Here the participant had to globally analyse a set of Isothermal Titration Calorimetry experiments using AFFINImeter and get the thermodynamic and structural parameters of the interaction between both molecules (the receptor protein and the ligand).
The participants in this contest had the opportunity to demonstrate their ability to propose the right model for a given binding isotherm as well as to get the corresponding parameters upon fitting using AFFINImeter. On their side, less experienced participants had the opportunity to:
During the last days we though it might be useful to help you throughout the fittings proposed for the contest. Therefore, we have prepared a video that explains, step by step, How to fit CURVE 1. IMPORTANTLY, this information will be of great help to solve step 2, the global fitting of CURVES 1-4.
The data proposed for the contest will remain available here, in case you want to learn more about it or to try it by yourself.
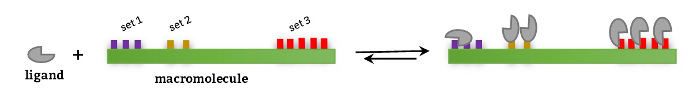
The interaction between two species, i.e. a protein and its ligand, is defined by means of the equilibria existing between free and bound species and the binding constant(s) associated to each equilibrium. This scenario can be described in terms or reaction schemes following two approaches:
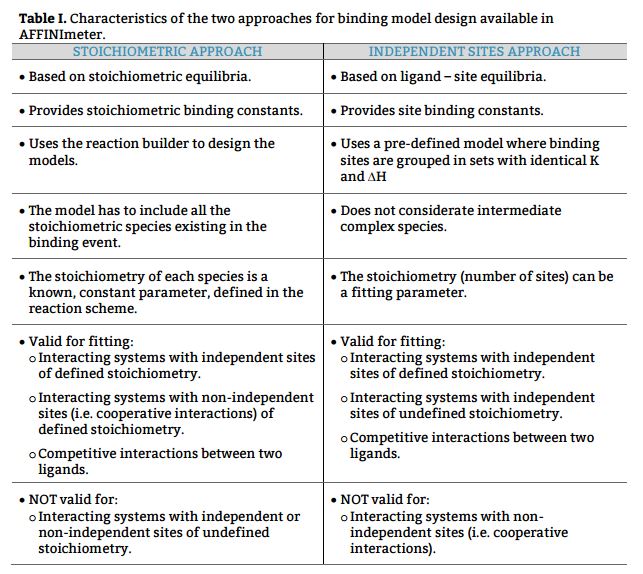
a) Based on equilibria between existing stoichiometric species, to obtain stoichiometric binding constants and
b) Based on equilibria between the ligand and specific interaction site(s) of the protein, to obtain site binding constants.
The understanding of both approaches/type of binding constants is key for a correct interpretation of the results after data analysis, in order to get key structural and mechanistic information of the binding event; i.e. the presence or absence of cooperative interactions when a ligand binds to a multivalent receptor.
The design of binding models for ITC curve fitting with AFFINImeter can be done following these two approaches, to perform analysis based on stoichiometric and/or site binding constants.
The scientific team of AFFINImeter has just released three NOTES regarding this subject to guide users into the right selection of binding model approach and a better understanding of stoichiometric vs site binding constants.
DOWNLOAD PDF FILES HERE:
Or visit the RESOURCES section of AFFINImeter web page where you find tutorials, webinars, cases of use, among others.
Isothermal titration calorimetry (ITC) is an extremely sensitive technique to assess for the formation/disruption of complex chemical/biological species in solution. During the last years, the increase in instrument sensitivity as well as the reduction of the sample concentration required to perform experiments, have made possible to expand the application range of ITC, which is expected to continue growing.
The amount and the quality of useful information that can be obtained from an ITC experiment depend on several factors including the purity of the samples, the concentration of the solutions prepared, the choice of injection volume and its length in time. The researcher handling the instrument is responsible for the appropiate selection of these variables as part of the experimental setup. They can be optimized on the basis of previous experience and also taking advantage of computational simulations. A key factor for this is that ITC is an incremental technique and so the results depend strongly on the injection volume employed to perform the experiment.
The Global fitting of multiple isotherms is one of the advanced tools that AFFINImeter offers to facilitate the analysis and interpretation of isothermal titration experiments and to expand the range of applications of this technique.
The following video tutorial describes the global fitting of three isotherms of a displacement assay describing, a receptor interacting with a tight ligand, with a weak ligand, or with both ligands simultaneously, in a competitive experiment where the ligands are mixed in the syringe of the ITC equipment.
If you want to know more about global fittings with AFFINImeter you can also download the case of use “Global Analysis in ITC Displacement Titrations with AFFINImeter” that describes a Displacement Titration Assay to determine the thermodynamics of HIV-protease with indinavir, a high-affinity binder, and with acetyl-pepstatin, a weaker ligand.
ITC displacement titrations offer an attractive alternative to standard assays when working with ultra-high or ultra-low- affinity interacting systems. The method requires the fitting of at least two isotherms that share various adjustable parameters. The case study exemplifies the potential advantages of using AFFINImeter in ITC displacement assays. The software offers unique advanced tools that enhance the robustness of the method and makes it more versatile, facilitating the acquisition of reliable thermodynamic data from ultra-high of ultra-low affinity systems. Thus, it opens a door for new applications of the displacement assay.
Competitive binding assays where two (or more) ligands bind to the same receptor have become common experiments in many research areas, from basic investigations to innovation in the pharmaceutical industry. These assays can be done in different formats, i.e. through a displacement assay where ligand “L1” is displaced by ligand “L2” from a preformed complex “L1-receptor” or via titration of a receptor solution with a mixture of L1 +L2. Either way, the competitive binding assay provides rich thermodynamic and structural information of the various binding events taking place during the course of the experiment. Thus, Isothermal Titration Calorimetry (ITC) competition assays performed in a displacement format have been revealed as an efficient tool for the quantitative analysis of very high- / low- interactions, with application in the field of fragment based drug screening (ref).
The versatility of the experimental setup in AFFINImeter-ITC permits the analysis of ITC competition experiments in its various formats. As an illustration, the following lines describe the analysis of an ITC isotherm resulting from a competitive experiment where a solution of a receptor in titrated with mixture of two competing ligands.
AFFINImeter contains a series of examples with which users can practice and learn the overall process of data fitting: from equipment and data uploading to fitting model design and data fitting.
In this post we will review an example of a competitive model fitting model used to analyze the experiment data of two ligands in the syringe competing for binding to the same receptor.
The AFFINImeter example “competitive binding model” illustrates an ITC experiment where two ligands, “A” and “B” compete with each other for binding to the receptor “M”.

This situation corresponds to a binding model consisting of three free species (A, B and M) two binding equilibria representing the interaction of M with A and M with B
The model was designed with the “reaction builder” and stored in “models”.The equipment used is decribed and stored in “equipments”. The dataseries is uploaded and stored in “dataseries”. When the dataseries is uploaded, the user has to complete the information relative to the equipment used and the species concentration. In this particular case (where there is a competitor “B”).
1- Go to PROJECT MANAGEMENT and create a new PROJECT an a new FIT SUBPROJECT.
2- Press Run button.
This Steps are described in the following Video Tutorial:
You can follow this tutorial in AFFINImeter, the Experimental Data and Binding model are stored in your own AFFINImeter account. If you hasn’t registered yet go to the AFFINImeter Software WebPage to get your account.
Ref: W. B. Turnbull, Divided we fall? Studying low-affinity fragments of ligands by ITC. GE Healthcare Life Sciences protocol, 2011, pp 1-11.
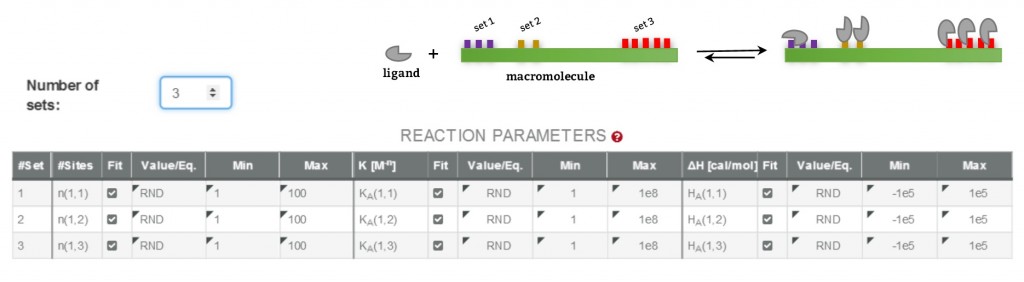
A successful Isothermal Titration Calorimetry (ITC) experiment requires the acquisition of high quality experimental data together with a careful analysis. Choosing the right binding model to fit the ITC isotherm is critical in order to get the true thermodynamic profile of the interaction. Often, the main limitation to achieve good results arises when the evaluation software lacks of the mathematical model that best describes our binding experiment. A good example is the case of a ligand binding to a macromolecule with multiple independent sites, i.e ligand – DNA interactions (1). Until now the readily available mathematical models to fit such experiments was limited to one or two sets of “n” independent identical sites; frequently, these models offer a poor description of the interaction due to the inherent higher complexity of the system, where many distinct binding equilibria coexist.
AFFINImeter ITC offers an unlimited number of user-defined binding models. Particularly, it counts with a feature to easily design models based on multiple independent binding sites. Here, a model with a number of sets of independent sites can be created with no limitation in the number of sets or sites. Noteworthy, the number of sites in each set can be considered as a fitting parameter throughout the data analysis. As an illustration, the following figure shows the reaction parameters of a model generated with AFFINImeter that describes a ligand binding to a receptor having 3 sets of sites, each set having an unknown number of sites. Fitting the experimental data to such model yields the microscopic association constant (K) and the change in enthalpy (ΔH) of the ligand binding to each site type, and the number of sites in each set (n).
These binding models, described by numerous variable parameters, may end up in an over-parameterized fitting function. Thus, the best strategy to achieve a robust and consistent analysis involves the global fitting of several ITC curves acquired under different experimental conditions. In this sense, AFFINImeter also supports global fitting of multiple isotherms wherein parameter linkage between curves is used to decrease the relative number of estimated parameters per experiment.
References:
(1) Methods 2007, 42, 162–172.
Use of precise Standard Reactions for Isothermal Titration Calorimeter Validation
Many published papers report inconsistent thermodynamic values of the same interactions between chemical reactants or macromolecular binding. One of the reasons for this discrepancies is the difficulty of repeating the same conditions in the ITC experiments (buffer, pH, concentrations, ionic strength, source of the materials…). But users start to be more aware that some systematic errors of the calorimeters may also have an important effect in the reported values.
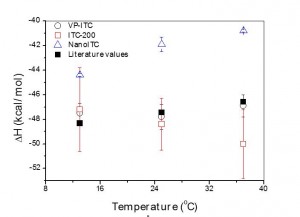
For instance, the interaction between 4-carboxybenzenesulfonamide and bovine carbonic anhydrase II is considered a standard reaction to be measured by ITC and its enthalpy has been measured by 14 operator using different calorimeters (1). The resulting value considering all these independent measurements is -10.4±2.5 kcal·mol-1. The error of the enthalpy is surprisingly high and significantly higher than those typically reported for ITC measurements.
Baranauskiené and co-workers (1) suggest the use of precise standard reactions for Isothermal Titration calorimeter validation after the calibration. The table below shows the series of chemical reaction they propose as standards where the enthalpy of binding has been determined to high precision and the reagents are readily available from commercial sources.
They also used these standards reactions to compare the results obtained with different micro calorimeter. Their study concluded that Microcal calorimeters are more reliable than TA Calorimeters; and the most recent Microcal ITC200 is less accurate than Microcal VP-ITC. Nano ITC-III calorimeter results were very reproducible, but enthalpy values were systematically underestimated. To learn more about Isothermal Titration Calorimeter validation,, visit the references from where this article was taken.
References:
(1) Int. J. Mol. Sci 2009, 10, 2752-2762.
(2) Handbook of proton Ionization Heats Wiley-Interscience: Hoboken, NJ, USA, 1979
Isothermal Titration Calorimetry Experiment Simulation
The Simulator tool available in AFFINImeter is completely free under registration. This is currently the only alternative to design complex Isothermal Titration Calorimetry (ITC) experiments. The Simulator allows plotting ITC curves (evolved heat as a function of the system concentration) together with a phase diagram of the different chemical species that are present in the solution regardless the complexity of the interaction mechanism between the involved molecules.
Avoid Trial and Error Assays
Using the AFFINImeter Simulator you will be able to pre-visualize the results of an experiment, provided that you have an approach for the interaction mechanism of your molecules and of the corresponding thermodynamical parameters. This tool will guide you in the optimization of the most advantageous combination of experimental parameters: the concentration and location (in the sample cell or in the syringe) of your compounds, the injection volume and the number of titrations; thus avoiding trial-and-error assays and saving time, reactants and money.
This tool is also useful to set the conditions under which the distribution of chemical species meet some special requirement (for instance, the solution dominated by a given chemical species). It can also be used for didactic purposes since it helps to illustrate how a chemical species can be displaced by another, to explain the difference between cooperative and non-cooperative processes or to explain the effect of endothermic and exothermic processes.
Applications in Drug Discovery
Isothermal Titration Calorimetry is a key technique in the development of drugs since it assess the affinity between molecules. The most typical application is to determine the free energy of interaction between proteins and inhibitors. The AFFINImeter simulator tool allows simulating the displacement of a weak ligand by a strong ligand as a function of the concentration of the compounds involved in the experiment.
Advantages of the Simulator
Introduce your personalized thermodynamic model directly in chemical language (reaction scheme) and an estimation for the corresponding thermodynamic parameters. Even the most advanced models are easy to implement. Through the model builder AFFINImeter offers an unlimited amount of thermodynamic models for Isothermal Titration Calorimetry data analysis. If the model required for your system is not available, please, do not hesitate to contact us and we will try to implement it.
Start Using the Simulator
The AFFINImeter Simulator is free under registration. To learn how to use it, please read this tutorial.
The Model Builder is one of the novel features of AFFINImeter. Through the model builder AFFINImeter offers an unlimited amount of thermodynamic models for ITC data analysis. The overall binding equilibria within the species involved in the experiments is easily drawn by the user directly in chemical language. Then AFFINImeter translates the resulting reaction scheme into robust binding models to be used to isotherm ITC simulation or to perform Isothermal Titration Calorimetry curve fitting.
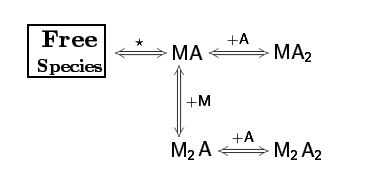
The model builder is a versatile tool, it allows to design models involving up to three different species (i.e. the case of two ligands that compete with each other to bind a macromolecule) and has the advantage to selectively place them in the syringe cell and/or in the calorimetric cell.
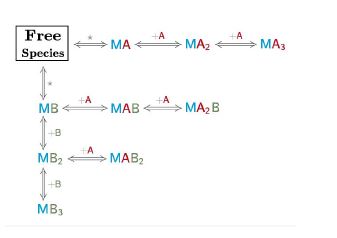
It also allows the design of models for dissociation, ranging from simple homodimers to higher-order oligomers. During the model construction no mathematical equations are required, once the whole set of binding interactions is defined by the user in the reaction builder, AFFINImeter internally generates the system of equations that define the reaction scheme proposed. The new model (reaction scheme and equations) is saved internally by AFFINImeter and listed in the user’s database so that can be utilized anytime so simulate or fit data.
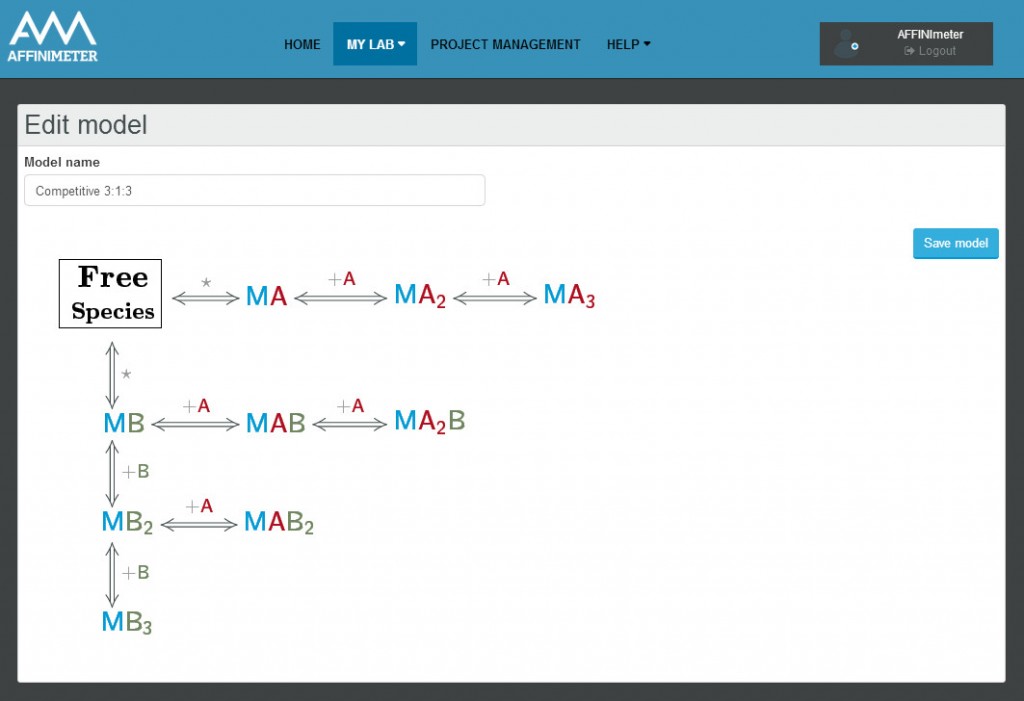
AFFINImeter-ITC offers an exclusive unlimited amount of personalized model families including
With this extensive offer of model families the user will be able to perform the thermodynamic characterization of a vast variety of biological and physicochemical processes from ITC measurements. A few examples of classical and new applications of ITC experiments that you can analyze with AFFINImeter are: