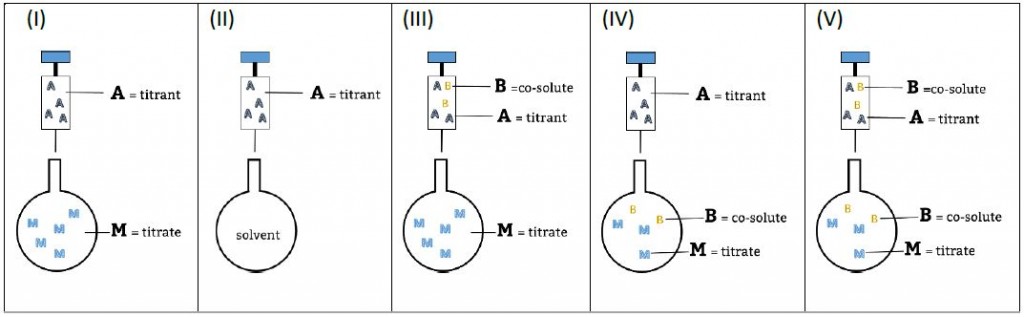
Experimental setups and nomenclature used in AFFINImeter
Representative binding models based on a Stoichiometric Equilibria approach
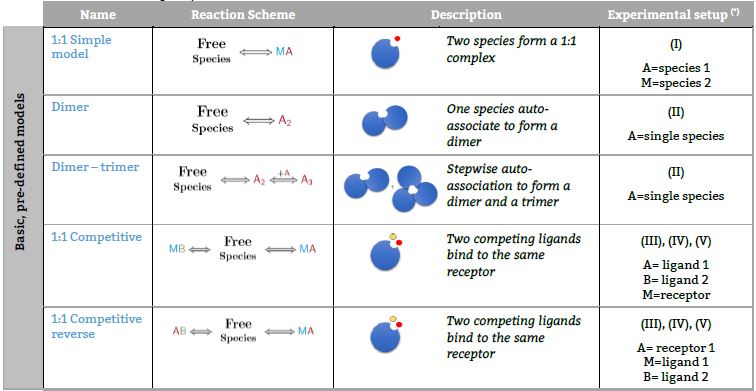
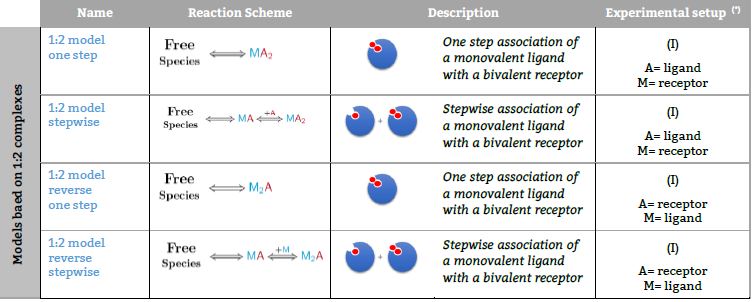
Representative binding models based on a Stoichiometric equilibria approach. Fitting of the ITC isotherm(s) to these models provide the Stoichiometric binding constant and the enthalpy change of each equilibrum involved in the reaction scheme.(*) The number in parenthesis,
Basic, predefined models
Models based on 1:2 complexes
Models based on autoassociation
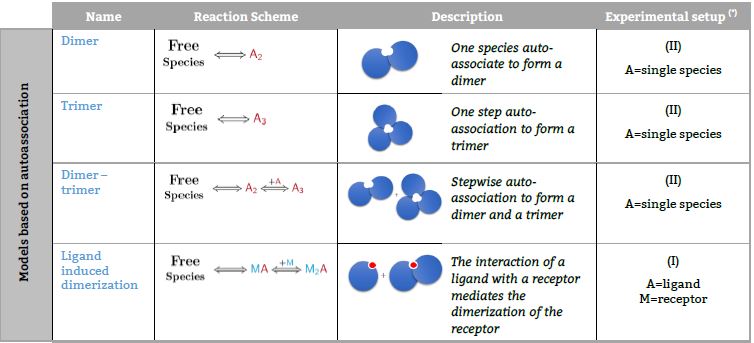
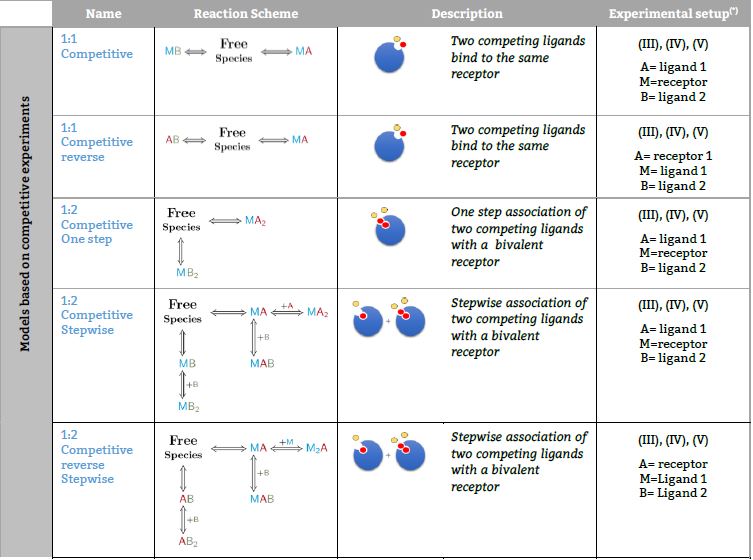
Models based on competitive experiments
Representative binding models based on an independent sites approach
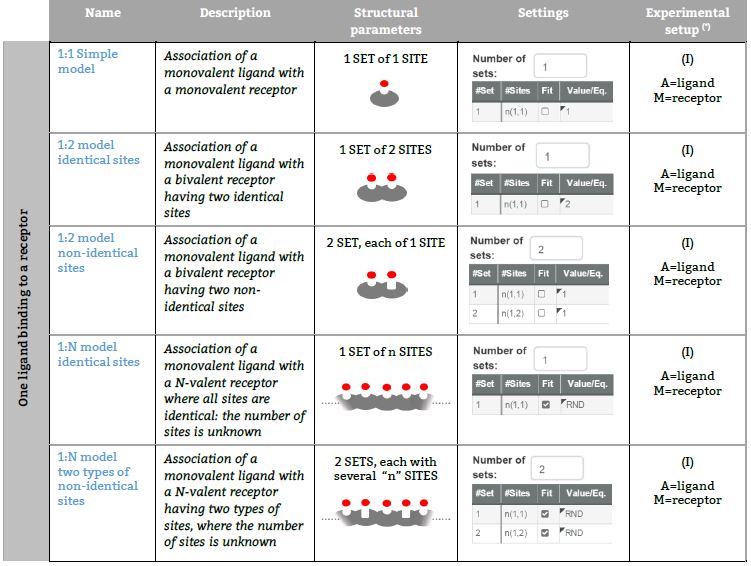
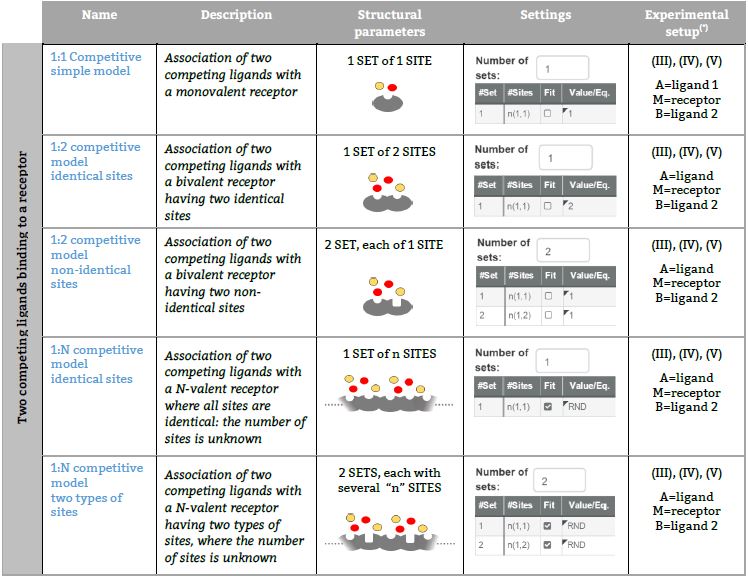
Representative binding models based on an independent sites approach Fitting of the ITC isotherm(s) to these models provide the Site binding constant and the enthalpy change of each ligand – site interaction. (*) The number in parenthesis, (I), (II), (III), (IV) or (V) refers to five ITC experimental setups, respectively (see TABLE I for a schematic description).
One ligand binding to a Receptor
Two competing ligands binding to a receptor
The scientific team of AFFINImeter has just released three NOTES regarding this subject to guide users into the right selection of binding model approach and a better understanding of stoichiometric vs site binding constants.
DOWNLOAD PDF FILES HERE:
- Stoichiometric and site constants: two approaches to analyze data with AFFINImeter
- The stoichiometric equilibria approach to design binding models with AFFINImeter
- The independent sites approach to design binding models with AFFINImeter
Or visit the RESOURCES section of AFFINImeter web page where you find tutorials, webinars, cases of use, among others.