Use of precise Standard Reactions for Isothermal Titration Calorimeter Validation
Many published papers report inconsistent thermodynamic values of the same interactions between chemical reactants or macromolecular binding. One of the reasons for this discrepancies is the difficulty of repeating the same conditions in the ITC experiments (buffer, pH, concentrations, ionic strength, source of the materials…). But users start to be more aware that some systematic errors of the calorimeters may also have an important effect in the reported values.
For instance, the interaction between 4-carboxybenzenesulfonamide and bovine carbonic anhydrase II is considered a standard reaction to be measured by ITC and its enthalpy has been measured by 14 operator using different calorimeters (1). The resulting value considering all these independent measurements is -10.4±2.5 kcal·mol-1. The error of the enthalpy is surprisingly high and significantly higher than those typically reported for ITC measurements.
Baranauskiené and co-workers (1) suggest the use of precise standard reactions for Isothermal Titration calorimeter validation after the calibration. The table below shows the series of chemical reaction they propose as standards where the enthalpy of binding has been determined to high precision and the reagents are readily available from commercial sources.
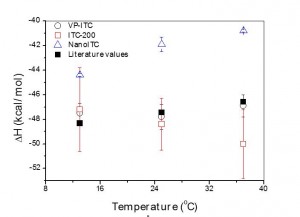
They also used these standards reactions to compare the results obtained with different micro calorimeter. Their study concluded that Microcal calorimeters are more reliable than TA Calorimeters; and the most recent Microcal ITC200 is less accurate than Microcal VP-ITC. Nano ITC-III calorimeter results were very reproducible, but enthalpy values were systematically underestimated. To learn more about Isothermal Titration Calorimeter validation,, visit the references from where this article was taken.
References:
(1) Int. J. Mol. Sci 2009, 10, 2752-2762.
(2) Handbook of proton Ionization Heats Wiley-Interscience: Hoboken, NJ, USA, 1979