Advantages of performing a global fitting of several Isothermal Titration Calorimetry isotherms
We have remarked the relevance of global fitting as an indispensable tool to perform a robust analysis of complex binding interactions. Actually, global fitting is a very advantageous tool for the analysis of simpler binding modes like standard 1:1 interactions and it can be used to identify the source of potential differences observed between experiment repeats.
Here we will see how to use global fitting together with the parameters rA and rM of AFFINImeter to identify the source of discrepancies between repeats of the same experiment, to ultimately end up with reliable results.
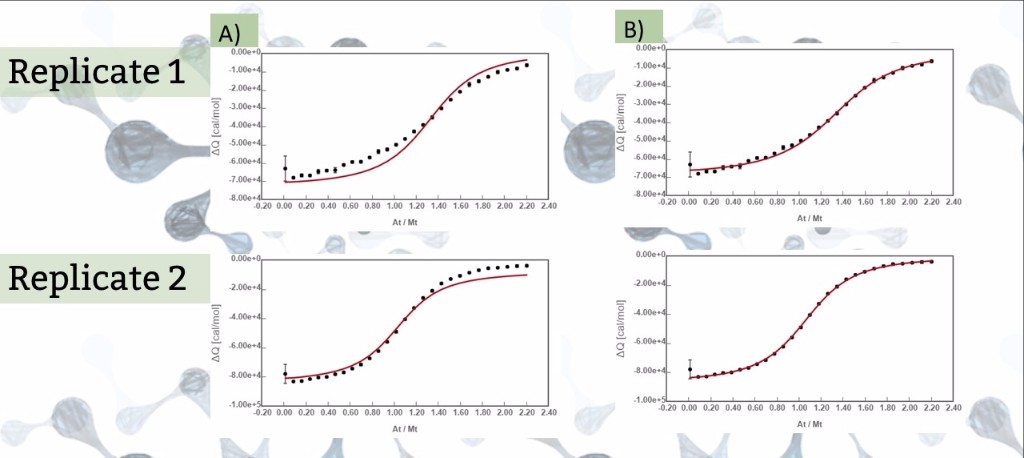
The isotherms of figure 1 are replicates of the same ITC experiment where a 1:1 interaction between the titrant (A) and the titrate (M) is monitored, but individual fitting of each isotherm to the simple model M + A ↔ MA (Figure 2) yield different KA (association constant) and ΔH values. In fact, from a visual inspection of the isotherms one can presume that the saturation degree reached in replicate 1 is less than in replicate 2 and that the mid titration point is below the expected stoichiometric equivalence point (At/Mt = 1), most probably because the titrant (A) and/or titrate (M) concentration was not accurately determined in this experiment.
Figure 1. A) Global fitting 2 where K, ΔH and rA are global parameters and rM is individual between isotherms. B) Global fitting 1 where KA, ΔH and rM are global parameters and rA is individual between isotherms.
A global fitting of the two isotherms was performed in which KA, ΔH and rA are shared fitting parameters amongst the isotherms; moreover, rM* is set as individual fitting parameter to check for potential deviations between nominal and true concentration of M in replicate 1; similarly, a global fitting was performed in which KA, ΔH and rM are shared parameters, and rA* is an individual fitting parameter to check for potential deviations between nominal and true concentration of A (figure 1).
Figure 2. Representation of the experiment using the AFFINImeter AM code.
Comparison of the two global fittings clearly shows that a good result is only achieved when deviations in the concentration of A is considered. The value of rA obtained indicates that the active concentration of A is 79% times the nominal concentration (table).
Table: Results obtained from global fitting 2.
This protocol using AFFINImeter has already been proven to be useful in drug discovery programs, working with ligand samples of inaccurate concentration.
We would like to thank Dr. Eric Ennifar (University of Strasbourg) for kindly providing the ITC experiments described herein.
*rM, rA, are scaling parameters employed to correct for potential differences between the nominal and true concentration of compounds M and A, respectively.